Industry
Made-in-Korea COVID-19 treatments ready to hit market
Jan. 14, 2021
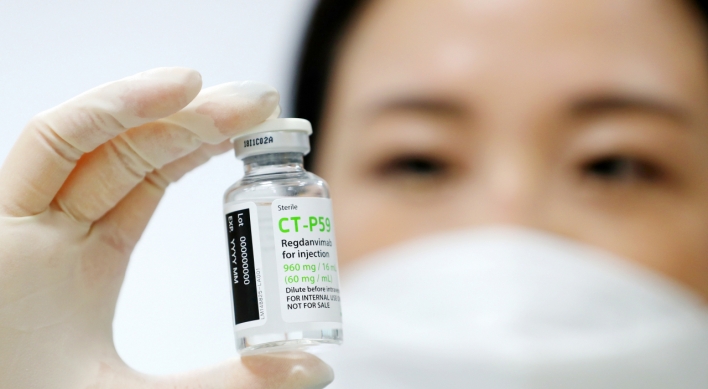
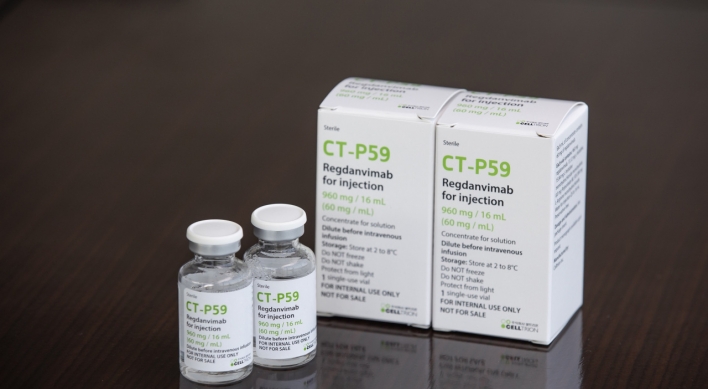
One company after another, South Korea’s homegrown biopharmaceuticals are nearing emergency use approval for their proposed COVID-19 treatments. A day after Celltrion reported positive top-line data from its antibody drug regdanvimab, or CT-P59, another local drugmaker, Chong Kun Dang, presented promising results on Thursday from its own drug, nafamostat, from a clinical phase 2 trial. Tested on over 100 people in Russia, 36 of them high-risk patients, nafamostat showed 61.1 percent cl







![[Herald Interview] Oral health care in the hands of consumers](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2021/01/17/20210117000017_0.jpg&u=20210118085825)





![[News Analysis] Is Korea prepared for vaccine rollout?](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2021/01/12/20210112000754_0.jpg&u=20210112180328)


![[Eye Plus] Gapyeong’s garden of fantastic lights](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2021/01/07/20210107000719_0.jpg&u=20210110091418)