
Wizbiosolutions CEO Lee Hyun-young (Wizbiosolutions)
Wizbiosolutions started in 2010 as a one-man company. A decade of research and development down the road, it has encountered the ultimate stressor that has catapulted its growth -- the global COVID-19 pandemic.
Wizbiosolutions’ portable diagnostics system, which enables field diagnostic test at airports and harbors, as well as room temperature storable reagent for polymerase chain reaction, have been key to the company’s successful Series A funding amounting to $10 million in October, according to Wizbiosolutions CEO Lee Hyun-young.
“There had been more business requests than we could handle in the first half of 2020,” said Lee, in an interview with The Korea Herald.
The company’s products are especially effective for speedy relief in low-infrastructure areas. Wizbiosolutions’ EZ-Buffer can extract DNA information from samples drawn from a person in five minutes, a big cut from the typical 20 minutes, according to Lee.
Added with the CrystalMix lyophilized reagent that can be stored and transported at room temperature, its technology can detect COVID-19 infection in a person in just 40 minutes, the CEO explained. Previously, similar endeavors required 2 1/2 hours.
This quick testing can be done on-site at transportation hubs -- while traditional PCR diagnostics systems cost over $45,000 and are as big as bulky multifunction printers for offices, Wizbiosolutions’ portable PCR systems are only a little bigger than the retro-style home telephones.
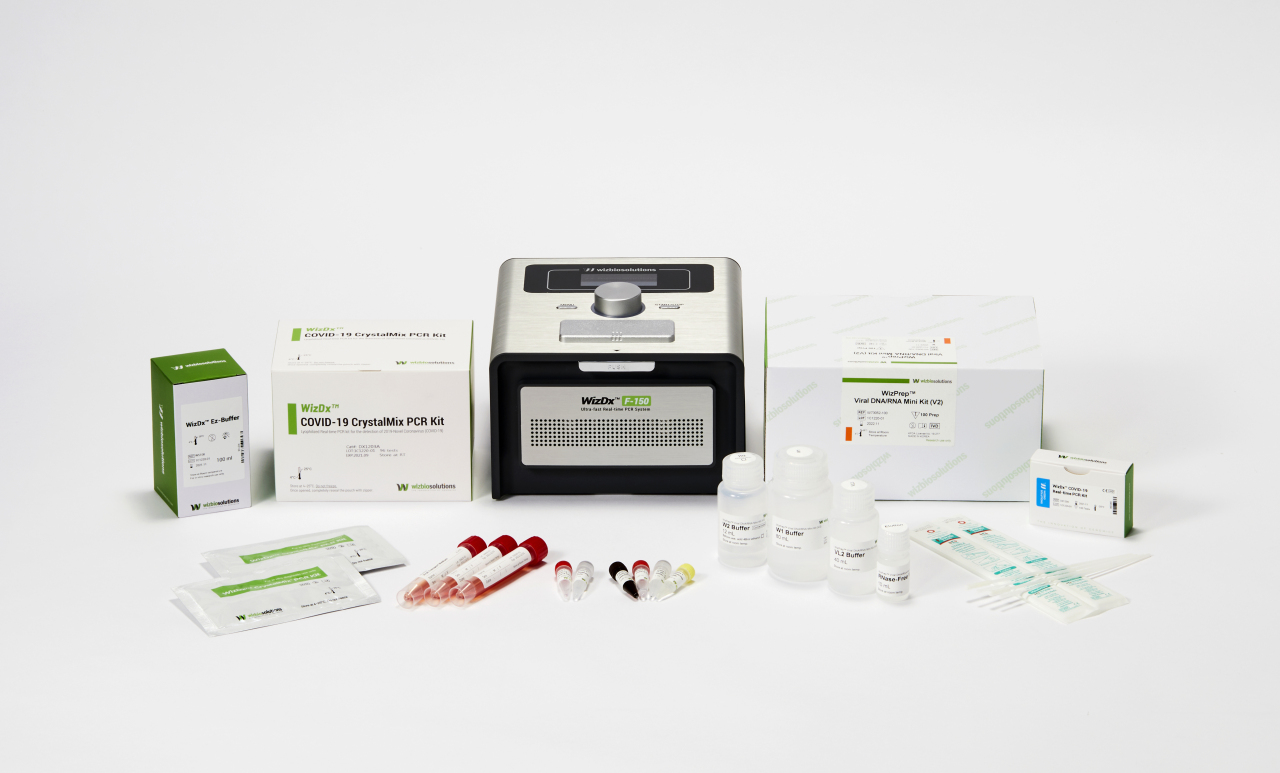
Main products from Wizbiosolutions (Wizbiosolutions)
Wizbiosolutions’s products not only reduce testing time. They also help decrease human errors by simplifying the steps, Lee said.
“Anyone, given basic training, can operate our real-time PCR kit. They don’t have to have a science background,” Lee said.
There is also the matter of price competitiveness. Wizbiosolutions makes its own source material, Lee said, which drastically cuts down the price of the end products compared to competitors relying on outsourced materials.
Already this year, Wizbiosolutions has exported more than 500,000 test kits to Indonesia, Vietnam, Iraq, Singapore, Poland and Romania.
With the newly earned investment, the company is increasing its facility in Seongnam, Gyeonggi Province, 20-fold. Within December, Wizbiosolutions will have a monthly output of 10 million test kits.
Regarding the sustainability of such an enlarged manufacturing facility, CEO Lee said, “We believe the demand for diagnostics kits will continue after the COVID-19. We not only make kits for COVID-19 assay, but are also developing kits for influenza, dengue, Middle East respiratory syndrome and malaria, which we plan to begin sales in 2021,” Lee said.
According to the Biotech Policy Research Center, the global market for in vitro diagnostic assay kits is anticipated to grow from $72 billion in 2019 to $127 billion in 2022.
“Wizbiosolutions will grow to rank among the global top 10 field molecular diagnostics firms,” said Lee.
By Lim Jeong-yeo (
kaylalim@heraldcorp.com)







![[From the Scene] Monks, Buddhists hail return of remains of Buddhas](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=645&simg=/content/image/2024/04/19/20240419050617_0.jpg&u=20240419175937)